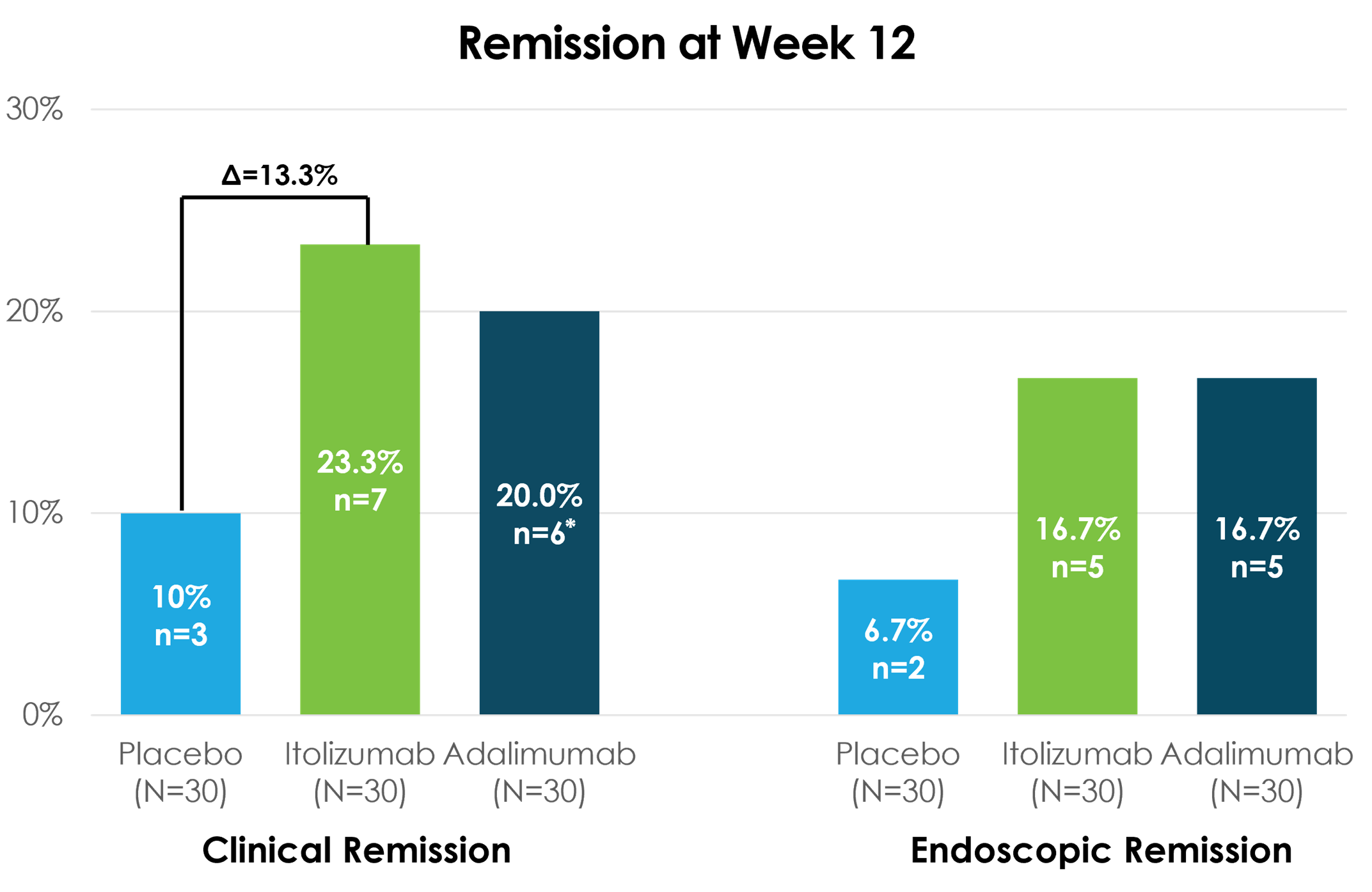
Itolizumab demonstrated clinical efficacy after 12 weeks of treatment, achieving a clinical remission rate of 23.3% compared to 20% for adalimumab and 10% for placebo
Itolizumab achieved key secondary endpoint of endoscopic remission of 16.7% compared to 16.7% for adalimumab and 6.7% for placebo
Itolizumab was generally well tolerated consistent with prior clinical experience
Bengaluru, Karnataka, India, February 6, 2025
Equillium, Inc. (Nasdaq: EQ), a clinical-stage biotechnology company leveraging a deep understanding of immunobiology to develop novel therapeutics to treat severe autoimmune and inflammatory disorders, and Biocon Limited (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceutical company, today announced positive topline results from the Phase 2 study evaluating itolizumab in the treatment of moderate to severe ulcerative colitis (UC).
The double-blinded, placebo- and active-controlled Phase 2 clinical study evaluated the safety and efficacy of itolizumab in biologic-naïve patients with moderate to severe active UC. A total of 90 patients were randomized 1:1:1 to receive itolizumab (fixed dose of 140 mg), placebo, or adalimumab (a global standard of care biologic treatment used as an active control) every two weeks for an initial 12-week treatment period. The primary endpoint of the study was clinical remission as defined by Total Mayo Score, and secondary endpoints included the proportion of participants who achieved clinical response and endoscopic remission (evaluated by central endoscopy). The study was co-sponsored by Equillium and Biocon Limited and conducted at multiple clinical trial sites in India. The design and conduct of the trial were a collaborative effort, with input from the gastroenterology community and leading global clinical and scientific experts in the field of inflammatory bowel disease (IBD).
“The CD6-ALCAM pathway is elevated in gastrointestinal inflammation and is associated with severity of disease in both ulcerative colitis and Crohn’s patients. As such, we are delighted with the strength of data across the primary and secondary endpoints of this Phase 2 study in moderate to severe ulcerative colitis patients,” said Dr. Stephen Connelly, chief scientific officer at Equillium. “Itolizumab was well tolerated and achieved a clinical remission rate of 23 percent despite an imbalance of more severe patients in the itolizumab arm compared to the other arms of the study. While these positive results add to itolizumab’s critical mass of safety and efficacy data across different patient populations, we are particularly encouraged by this data in the context of our Phase 3 EQUATOR study in acute graft-versus-host disease, where lower gastrointestinal pathogenesis is a key driver of mortality.”
“Itolizumab demonstrated proof of concept with a meaningful effect size – comparable to biologic standard of care adalimumab – in this Phase 2 study in subjects with moderate to severe ulcerative colitis,” said Dr. Brian Feagan, Professor of Medicine at the Schulich School of Medicine & Dentistry at the University of Western Ontario. “Itolizumab represents a novel selective immune modifying mechanism of action with great potential in a treatment paradigm needing differentiation and improved outcomes for patients.”
Summary of Key Study Results
Baseline demographics of the study included a median age of 39 years, relatively equal proportions of male and female subjects evenly distributed among study arms, and a mean weight of 58 kilograms.
Baseline disease severity of the study was greater in the itolizumab arm, where 23% of patients were classified as severe (Total Mayo Score of 11) versus 0% in the placebo and adalimumab arms, and 66% had left-sided colitis versus 30% and 43% in the placebo and adalimumab arms, respectively.
The primary endpoint of the study was clinical remission, defined as Total Mayo Score of ≤ 2 with no individual sub-score greater than 1 at Week 12. Secondary endpoints included the proportion of participants who achieved clinical response (per Total Mayo Score) and endoscopic remission (evaluated by central endoscopy) at Week 12 and Week 24. Additional data is expected to be presented at a future scientific conference during 2025.
- 23.3% clinical remission in the itolizumab arm at 12 weeks (primary endpoint) vs. 20% in adalimumab* vs. 10% in placebo
- 63.3% clinical response in the itolizumab arm at 12 weeks vs. 60% in adalimumab vs. 46.7% in placebo
- 16.7% endoscopic remission in the itolizumab arm at 12 weeks vs. 16.7% in adalimumab vs. 6.7% in placebo
- Itolizumab was generally well tolerated, and no safety signal was observed