Business Highlights
GENERICS: APIs & Generic Formulations
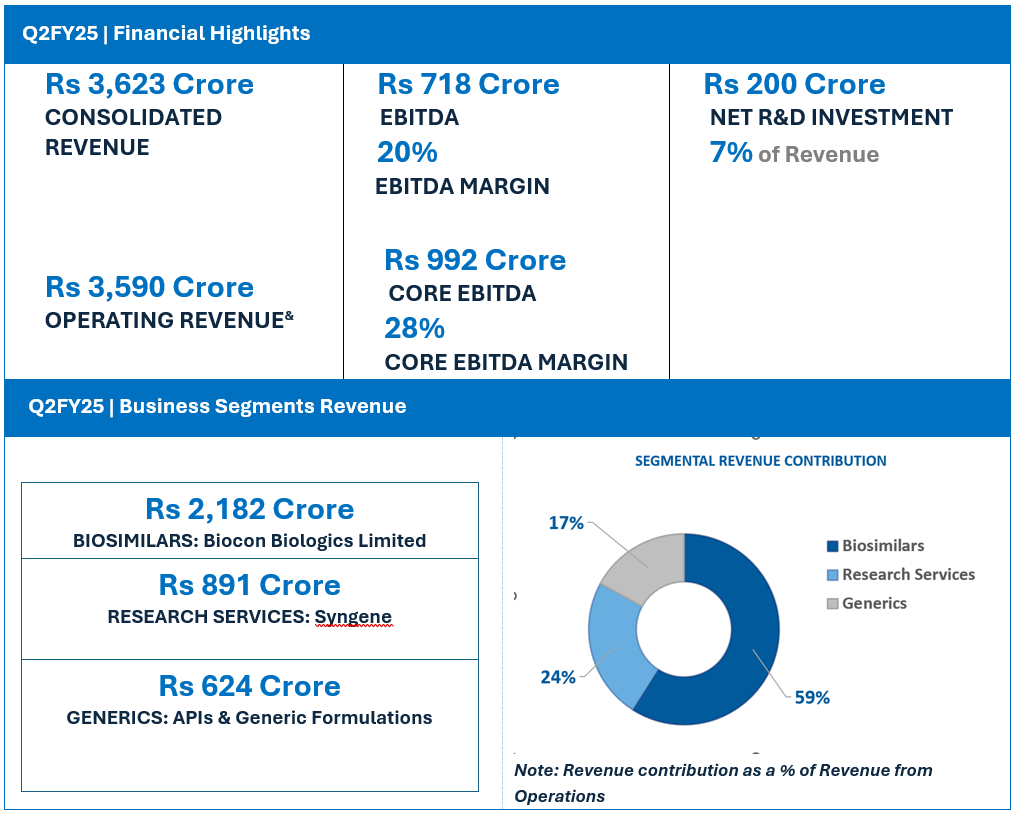
- Q2FY25 Revenue at Rs 624 Crore, down 8% YoY
Business Performance
During the quarter, the Company obtained approvals in the U.S. for Sacubitril + Valsartan tablets used in the treatment of chronic heart failure, and for Daptomycin for injection to treat bacterial infections. Approvals were also received for Micafungin 50mg an 100mg powder in the UK and EU, for Posaconazole DR tablets in the UAE and for Tacrolimus capsules in India.
The business secured a tender for Everolimus tablets in a MoW market, with supplies expected to commence in Q3FY25. Continuing its regional expansion strategy, the business entered into two new partnerships.
A licensing and supply agreement was signed with Tabuk Pharmaceutical for commercializing its GLP-1 products for both diabetes and chronic weight management in select countries within the Middle East. Biocon will develop and manufacture the products, while Tabuk will hold the marketing authorization rights and be responsible to register, import and promote them in the region.
Subsequently, in October, Biocon entered into an exclusive distribution and supply agreement with a leading speciality pharmaceutical company in Brazil for the commercialization of its vertically integrated drug product, Liraglutide (gVictoza® and gSaxenda®). Biocon will be responsible for regulatory approval, manufacturing and supply of the product, while the partner will commercialize it in Brazil.
Regulatory Inspections
Two separate U.S. FDA surveillance inspections of Biocon’s API facilities in Bengaluru (Sites 1 & 2) concluded in September with a few observations. The Company has submitted individual Corrective and Preventive Action (CAPA) plans to the agency and is confident of addressing these expeditiously. During the quarter, Biocon has received Establishment Inspection Reports (EIRs) for both its API sites in Visakhapatnam (Sites 5 and 6) from the U.S. FDA, following inspections conducted in June 2024.
BIOSIMILARS: Biocon Biologics
- Q2FY25 Revenue at Rs 2,182 Crore, Up 19% YoY on a Like-for-Like^ Basis
- Served 5+ million patients (MAT September 2024 basis)##
^After adjusting Q2FY24 revenue for Branded Formulations, India business
##12-month moving annual patient population (October 2023 to September 2024)
Business Performance
Q2FY25
Biocon Biologics’ Q2FY25 revenue at Rs 2,182 crore reported a YoY growth of 19% on a like-for-like basis after adjusting Q2FY24 revenue** for Branded Formulations, India business.
Core EBITDA stood at Rs 691 crore, with Core EBITDA margin at 32%. It reported EBITDA of Rs 469 crore, representing EBITDA margins of 21%. Adjusting for the forex impact of the appreciation of the Japanese yen versus the U.S. dollar, EBITDA was Rs 550 crore with a margin of 25%, reflecting the strong underlying profitability of the core business.
Notes: **Q2FY24 Revenue included Branded Formulations India sales which is not a part of Q2FY25 revenue.
Strategic Refinancing
Biocon Biologics successfully refinanced its long-term debt of US$1.1 billion through a combination of US$800 million U.S. dollar-denominated bonds and a new syndicated loan facility. This strategic re-financing will help improve the Company’s liquidity profile, provide enhanced financial flexibility, strengthen its capital structure and allow it to re-deploy investments into the business to fuel long-term growth. The bonds have been listed on the Singapore Stock Exchange, and it is the first U.S. dollar-denominated bond issuance by any biopharmaceutical company in Asia-Pacific, as well as the largest high yield debut bond issuance from India in the past 10 years.
Advanced Markets
North America@
Biocon Biologics has positioned itself as a strategic player among customers, payors, and patients in North America.
Its Oncology franchise, comprising Ogivri® (bTrastuzumab) and Fulphila® (bPegfilgrastim), is witnessing robust demand with a marked YoY increase in market share. The market share for Ogivri® increased to 18% from 11%, while Fulphila® rose to 21% from 15% (YoY). Market share for unbranded bGlargine and Semglee® surpassed 15%, which includes share from closed-door pharmacy networks and government business, on the back of a steady demand.
In July, the Company moved into its new North America headquarters in Bridgewater, New Jersey, and marked its first anniversary as an independent commercial organization in the United States and Canada.
Europe@ and JANZ
Biocon Biologics reported steady market shares in Europe at a regional level with Germany and France continuing to drive value. Strong growth across key markets and products has allowed the Company to expand patient access. Hulio® (bAdalimumab) retained its market-leading position in Germany with an 18% market share and 11% in France. The Company has made significant progress in expanding its footprint, with strong market growth in the UK and the Mediterranean cluster, including Italy and Spain.
The Company obtained approvals for bAspart and bEtanercept in New Zealand during the quarter.
@Market shares based on IQVIA Q2 CY2024 data.
The data presented hereunder inter alia volumes, projections, market share, is based solely on our study, interpretation and conclusion derived through analysis of different data sets from varied sources inter alia IQVIA.
Emerging Markets
The Emerging Markets (EMs) business continues to expand patient reach, securing market leading shares in several key countries such as 74% for bTrastuzumab and 86% for bBevacizumab in South Africa. The Company continues to witness strong demand for its portfolio especially for insulins in Mexico and for bAdalimumab and bEtanercept in Saudi Arabia. Biocon Biologics has also seen an uptick in market shares across its self-led markets, which are a strategic priority for the Company.
During the second quarter, 15 launches of key commercialized products were accomplished across AFMET and LATAM regions such as bBevacizumab and bPegfilgrastim in Saudi Arabia. These new launches will be the key growth drivers, going forward.
The Company received regulatory approvals for five of its products, viz., bBevacizumab, bEtanercept, bAdalimumab, bAspart and rh-Insulin in several countries in the AFMET and LATAM regions.
To strengthen the business further, the Company is working on localization in multiple countries in the AFMET region and exploring new partnerships in the APAC region.
Regulatory Inspections
In terms of facility inspections, the U.S.FDA has classified Biocon Biologics’ Drug Substance facility at Biocon Campus, Bengaluru as Voluntary Action Initiated (VAI). This inspection was held in February 2024 and pertains to the supply of rh-insulin drug substance to the United States.
The Company has submitted a comprehensive Corrective and Preventive Action (CAPA) plan to the U.S. FDA following the cGMP inspection at its insulins manufacturing facility in Malaysia in September 2024. Another CAPA plan has been submitted to the agency related to the inspection of its manufacturing facility at Biocon Park, Bengaluru in July 2024.
The Company is confident of addressing all the observations expeditiously and does not expect the outcome of these inspections to impact the supply of its commercialized products. Furthermore, these sites are cGMP certified by several other leading global regulators including the Europe Medicines Agency (EMA), and Health Canada.
Progress in Pipeline
The European Medicines Agency (EMA) has validated Biocon Biologics’ regulatory filling for bDenosumab and the Company is on track to complete filings in several other markets later this year.
To strengthen its immunology franchise, the Company has signed a settlement and license agreement with Janssen and J&J, clearing the way for the commercialization of Yesintek (bUstekinumab), a proposed biosimilar to Stelara®, in Europe, the UK, Canada, and Japan upon regulatory approval.
Scientific Publications & Presentations
At the European Academy of Dermatology and Venereology (EADV) 2024 Congress in Amsterdam, the Company presented results from two separate pivotal Phase 3 clinical studies in patients with chronic plaque psoriasis supporting interchangeability between biosimilar Adalimumab-fkjp (low concentration) and the Adalimumab reference product (high concentration); and between Bmab 1200 (Yesintek) and the Ustekinumab reference product, establishing bio-equivalence, efficacy and comparable safety.
Biocon Biologics has released a report in collaboration with Clarivate that highlights pathways to increase adoption of biosimilars in low- and middle-income countries (LMICs). A peer-reviewed article based on this study titled “Increasing Adoption of Quality-Assured Biosimilars to Address Access Challenges in Low- and Middle-Income Countries” has been published in the prestigious Generics and Biosimilars Initiative (GaBI) Journal.